Subtitles & vocabulary
25. C-13 and 2D NMR. Electrophilic Aromatic Substitution
00
Cheng-Hong Liu posted on 2015/01/26Save
Video vocabulary
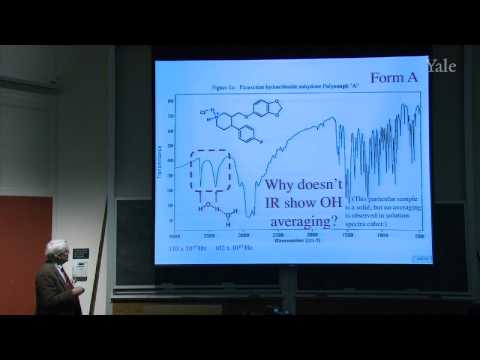
average
US /ˈævərɪdʒ, ˈævrɪdʒ/
・
UK /'ævərɪdʒ/
- Noun (Countable/Uncountable)
- Total of numbers divided by the number of items
- Transitive Verb
- To add numbers then divide by the number of items
A2TOEIC
More couple
US /ˈkʌpəl/
・
UK /'kʌpl/
- Transitive Verb
- To join something to something else
- (Two animals) to engage in sexual relations
- Noun (Countable/Uncountable)
- Two people in a romantic relationship
- Two of something; two people; a pair
A2
More field
US /fild/
・
UK /fi:ld/
- Noun
- Area of study, such as physics or biology
- Piece of land used to grow crops/raise animals
- Transitive Verb
- To respond to something or answer a question
- To catch or stop a ball during a game
A1TOEIC
More shift
US /ʃɪft/
・
UK /ʃɪft/
- Verb (Transitive/Intransitive)
- To change in position or direction
- To move something from one place to another
- Noun (Countable/Uncountable)
- A change in a persons plans, opinions or beliefs
- Period of work starting at a certain time
A2
More Use Energy
Unlock Vocabulary
Unlock pronunciation, explanations, and filters
