Subtitles & vocabulary
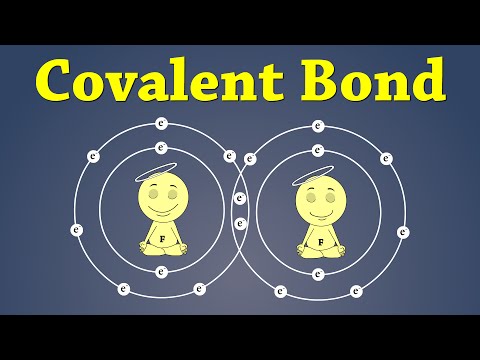
Covalent Bonding | #aumsum
00
AumSum posted on 2019/04/16Save
Video vocabulary
subscribe
US /səbˈskraɪb/
・
UK /səb'skraɪb/
- Verb (Transitive/Intransitive)
- To regularly pay to receive a service
B1TOEIC
More achieve
US /əˈtʃiv/
・
UK /ə'tʃi:v/
- Transitive Verb
- To succeed in doing good, usually by working hard
- To succeed in reaching a particular goal, status, or standard, often after effort or perseverance.
A2TOEIC
More represent
US /ˌrɛprɪˈzɛnt/
・
UK /ˌreprɪ'zent/
- Transitive Verb
- To depict art objects, figures, scenes; to portray
- To show or describe something in a particular way
A2TOEIC
More Use Energy
Unlock Vocabulary
Unlock pronunciation, explanations, and filters
