Subtitles & vocabulary
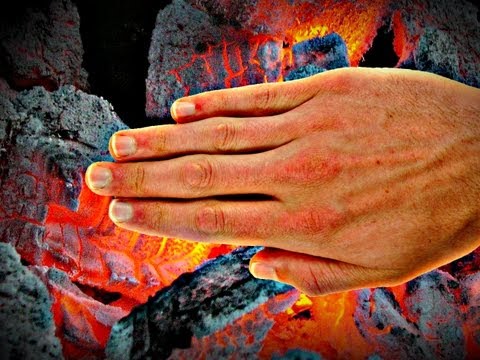
Can Humans Really Feel Temperature?
00
VoiceTube posted on 2013/08/22Save
Video vocabulary
stuff
US /stʌf/
・
UK /stʌf/
- Uncountable Noun
- Generic description for things, materials, objects
- Transitive Verb
- To push material inside something, with force
B1
More physical
US /ˈfɪzɪkəl/
・
UK /ˈfɪzɪkl/
- Countable Noun
- Health check at the doctors' or hospital
- Adjective
- Concerning the body of a person
- Concerning things that can be seen or touched
A2
More trick
US /trɪk/
・
UK /trɪk/
- Transitive Verb
- To fool someone in order to obtain a result
- To playfully tease or fool to make someone laugh
- Noun (Countable/Uncountable)
- Act of trying to fool someone
- Quick or skillful way of doing something
A2
More experience
US /ɪkˈspɪriəns/
・
UK /ɪk'spɪərɪəns/
- Countable Noun
- Thing a person has done or that happened to them
- An event at which you learned something
- Noun (Countable/Uncountable)
- Knowledge gained by living life, doing new things
- Previous work in a particular field.
A1TOEIC
More Use Energy
Unlock Vocabulary
Unlock pronunciation, explanations, and filters
