Subtitles & vocabulary
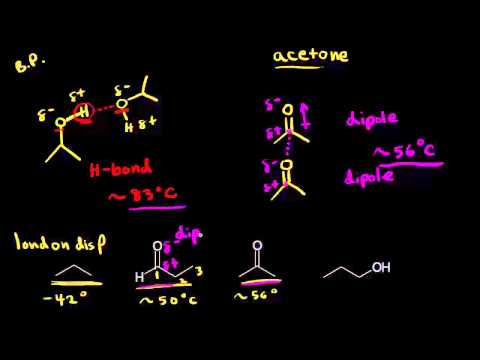
Physical properties of aldehydes and ketones
00
吳思賢 posted on 2016/06/23Save
Video vocabulary
negative
US /ˈnɛɡətɪv/
・
UK /'neɡətɪv/
- Noun
- The opposite to a positive electrical charge
- In grammar, containing words such as 'no' or 'not'
- Adjective
- Being harmful, unwanted or unhelpful
- In mathematics, being less than zero
A2
More positive
US /ˈpɑzɪtɪv/
・
UK /ˈpɒzətɪv/
- Adjective
- Showing agreement or support for something
- Being sure about something; knowing the truth
- Noun
- A photograph in which light areas are light and dark areas are dark
A2
More point
US /pɔɪnt/
・
UK /pɔɪnt/
- Noun (Countable/Uncountable)
- An item to be discussed
- Small spot or dot
- Intransitive Verb
- To face a certain direction, e.g. north
A1TOEIC
More Use Energy
Unlock Vocabulary
Unlock pronunciation, explanations, and filters
