Subtitles & vocabulary
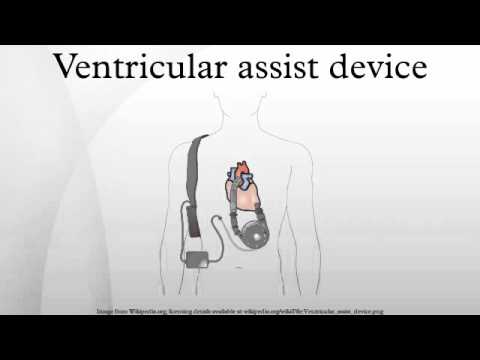
Ventricular assist device
00
Ting Huang posted on 2016/05/17Save
Video vocabulary
failure
US /'feɪljər/
・
UK /ˈfeɪljə(r)/
- Noun
- When things go wrong; lack of function
- Act or result of not achieving your goals
A1TOEIC
More therapy
US /ˈθɛrəpi/
・
UK /'θerəpɪ/
- Noun (Countable/Uncountable)
- Treatment to help cure an illness
- Psychological counseling to help resolve personal or emotional problems.
B2
More trial
US /ˈtraɪəl, traɪl/
・
UK /ˈtraɪəl/
- Noun (Countable/Uncountable)
- Hearing and judgment of a case in court
- Act or process of testing or experimenting
- Transitive Verb
- To be made or done as a test or experiment
A2TOEIC
More heart
US /hɑrt/
・
UK /hɑ:t/
- Noun (Countable/Uncountable)
- A feeling of care for others; compassion
- Courage, confidence, and emotional strength
A1
More Use Energy
Unlock Vocabulary
Unlock pronunciation, explanations, and filters
