Subtitles & vocabulary
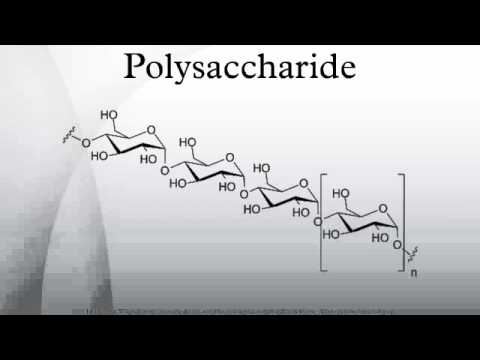
Polysaccharide
00
VoiceTube posted on 2016/03/12Save
Video vocabulary
structure
US /ˈstrʌk.tʃɚ/
・
UK /ˈstrʌk.tʃə/
- Noun (Countable/Uncountable)
- The way in which the parts of a system or object are arranged or organized, or a system arranged in this way
- A building or other man-made object.
- Transitive Verb
- To plan, organize, or arrange the parts of something
A2TOEIC
More bacteria
US /bækˈtɪriə/
・
UK /bæk'tɪərɪə/
- Noun (plural)
- The very small creatures that can cause disease
B2
More cell
US /sɛl/
・
UK /sel/
- Countable Noun
- Smallest unit of living things in biology
- Group of people - often from a secret organization
A2
More digest
US /daɪˈdʒest/
・
UK /daɪˈdʒest/
- Verb (Transitive/Intransitive)
- To convert food into energy in your stomach
- To think over facts, news etc.; take in information
- Noun
- A summary, as of the news
B2
More Use Energy
Unlock Vocabulary
Unlock pronunciation, explanations, and filters
