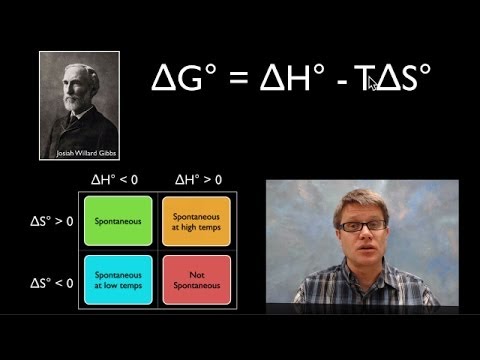
Subtitles & vocabulary
Using Gibbs Free Energy
00
李育真 posted on 2015/12/29Save
Video vocabulary
process
US /ˈprɑsˌɛs, ˈproˌsɛs/
・
UK /prə'ses/
- Transitive Verb
- To organize and use data in a computer
- To deal with official forms in the way required
- Noun (Countable/Uncountable)
- Dealing with official forms in the way required
- Set of changes that occur slowly and naturally
A2TOEIC
More figure
US /ˈfɪɡjɚ/
・
UK /ˈfiɡə/
- Verb (Transitive/Intransitive)
- To appear in a game, play or event
- To calculate how much something will cost
- Noun
- Your body shape
- Numbers in a calculation
A1TOEIC
More negative
US /ˈnɛɡətɪv/
・
UK /'neɡətɪv/
- Noun
- The opposite to a positive electrical charge
- In grammar, containing words such as 'no' or 'not'
- Adjective
- Being harmful, unwanted or unhelpful
- In mathematics, being less than zero
A2
More positive
US /ˈpɑzɪtɪv/
・
UK /ˈpɒzətɪv/
- Adjective
- Showing agreement or support for something
- Being sure about something; knowing the truth
- Noun
- A photograph in which light areas are light and dark areas are dark
A2
More Use Energy
Unlock Vocabulary
Unlock pronunciation, explanations, and filters
