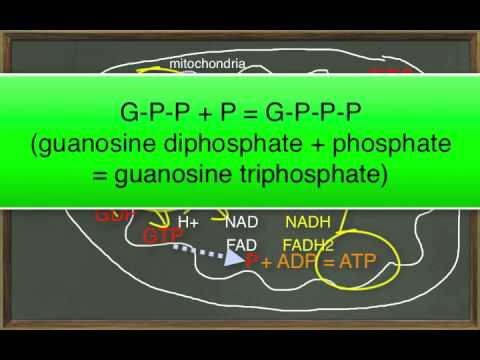
Subtitles & vocabulary
Chapter 25 Module 2 Glycolysis, TCA Cycle, and ETS
00
Cheng-Hong Liu posted on 2015/04/26Save
Video vocabulary
process
US /ˈprɑsˌɛs, ˈproˌsɛs/
・
UK /prə'ses/
- Transitive Verb
- To organize and use data in a computer
- To deal with official forms in the way required
- Noun (Countable/Uncountable)
- Dealing with official forms in the way required
- Set of changes that occur slowly and naturally
A2TOEIC
More call
US /kɔl/
・
UK /kɔ:l/
- Noun
- A order or request for action
- The sound an animal makes, often when in danger
- Verb (Transitive/Intransitive)
- To make a request or order for action
- To visit a place or person for a short time
A1
More system
US /ˈsɪstəm/
・
UK /'sɪstəm/
- Noun (Countable/Uncountable)
- Set of organized, planned ideas that work together
- A set of principles or procedures according to which something is done; an organized scheme or method.
- Adjective
- Working in an organized, logical way
A1TOEIC
More Use Energy
Unlock Vocabulary
Unlock pronunciation, explanations, and filters
