Subtitles & vocabulary
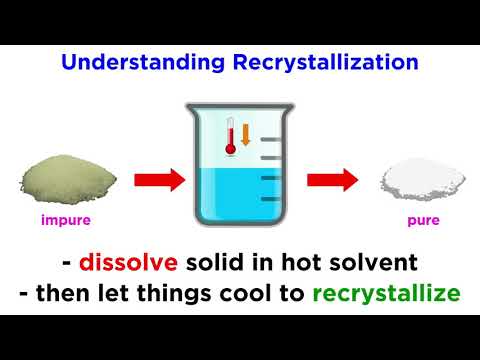
Recrystallization
00
ウィリアム オ ルウェル posted on 2021/06/25Save
Video vocabulary
process
US /ˈprɑsˌɛs, ˈproˌsɛs/
・
UK /prə'ses/
- Transitive Verb
- To organize and use data in a computer
- To deal with official forms in the way required
- Noun (Countable/Uncountable)
- Dealing with official forms in the way required
- Set of changes that occur slowly and naturally
A2TOEIC
More inevitable
US /ɪnˈɛvɪtəbəl/
・
UK /ɪnˈevɪtəbl/
- Adjective
- That must happen; certain to happen
- Sure to occur or happen
- Noun (Countable/Uncountable)
- A situation that is unavoidable
- Things that cannot be avoided
A2
More extremely
US /ɪk'strimlɪ/
・
UK /ɪkˈstri:mli/
- Adverb
- In a way that is much more than usual or expected
- Remarkably; unusually.
B1
More properly
US /ˈprɑːpərli/
・
UK /ˈprɔpəlɪ/
- Adverb
- In an appropriate or correct manner
- In a way that is suitable or appropriate.
A2
More Use Energy
Unlock Vocabulary
Unlock pronunciation, explanations, and filters
