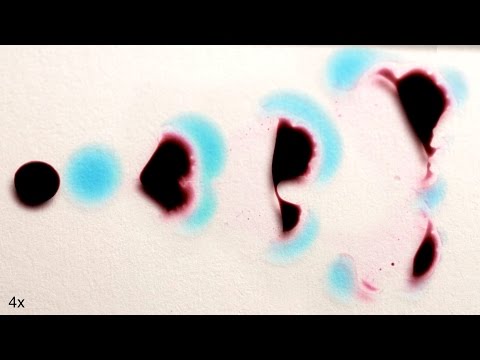
Subtitles & vocabulary
These Liquids Look Alive!
00
林宜悉 posted on 2020/03/28Save
Video vocabulary
constantly
US /ˈkɑnstəntlɪ/
・
UK /ˈkɒnstəntli/
- Adverb
- Frequently, or without pause
- In a way that is unchanging or faithful
B1
More essentially
US /ɪˈsenʃəli/
・
UK /ɪˈsenʃəli/
- Adverb
- Basically; (said when stating the basic facts)
- Used to emphasize the basic truth or fact of a situation.
A2
More permanent
US /ˈpɚmənənt/
・
UK /'pɜ:mənənt/
- Adjective
- Lasting forever; not temporary or changing
- Intended to last or remain for an unlimited period
- Noun
- A person who is a permanent employee.
- A chemical treatment to create lasting curls or waves in the hair.
B1TOEIC
More fascinating
US /ˈfæsəˌnetɪŋ/
・
UK /ˈfæsɪneɪtɪŋ/
- Transitive Verb
- To attract or interest greatly
- To hold someone captive with a gaze or other means.
- Adjective
- Having your attention fixated as though by a spell
B1
More Use Energy
Unlock Vocabulary
Unlock pronunciation, explanations, and filters
